Samacheer Kalvi 12th Chemistry Solutions Chapter 1 Metallurgy guide
12th Chemistry Chapter 1 Metallurgy Questions and Answers, Notes Pdf, Samacheerguide 12th Chemistry Solutions Guide Pdf helps you to revise the complete Tamilnadu State Board New Reduced Syllabus and get good marks in your examinations.
Tamilnadu Samacheer Kalvi 12th Chemistry Solutions Chapter 1 Metallurgy
Tamilnadu state board Samacheer Kalvi 12th Chemistry Metallurgy TextBook Evalution
I. Choose the correct answer.
Question 1.
Bauxite has the composition ………………
(a) Al₂O₃
(b) Al₂O₃.nH₂O
(c) Fe₂O₃.2H₂O
(d) None of these
Answer:
(b) Al₂O₃.nH₂O
Question 2.
Roasting of sulphide ore gives the gas (A). (A) is a colourless gas. Aqueous solution of (A) is acidic. The gas (A) is ………………
(a) CO₂
(b) SO₃
(c) SO₂
(d) H,S
Answer:
(c) SO₂
Question 3.
Which one of the following reaction represents calcination?
(a) 2Zn + O₂ → 2ZnO
(b) 2ZnS + 3O₂ → 2ZnO + 2SO₂
(c) MgCO₃ → MgO + CO₂
(d) Both (a) and (c)
Answer:
(c) MgCO₃ → MgO + CO₂
Question 4.
The metal oxide which cannot be reduced to metal by carbon is ………………
(a) PbO
(b) Al₂O₃
(C) ZnO
(d) FeO
Answer:
(b) Al₂O₃
Question 5.
Which of the metal is extracted by Hall-Herold process?
(a) Al
(b) Ni
(c) Cu
(d) Zn
Answer:
(a) Al
Question 6.
Which of the following statements, about the advantage of roasting of sulphide ore before reduction is not true?
(a) ∆Gfᵒ of sulphide is greater than those for CS₂ and H₂S.
(b) ∆Gᵣºis negative for roasting of sulphide ore to oxide.
(c) Roasting of the sulphide to its oxide is thermodynamically feasible.
(d) Carbon and hydrogen are suitable reducing agents for metal sulphides.
Answer:
(d) Carbon and hydrogen are suitable reducing agents for metal sulphides.
Question 7.
Match items in column -1 with the items of column – II and assign the correct code:
Answer:
(c) A – (iv), B – (ii), C – (iii), D – (i)
Question 8.
Wolframite ore is separated from tinstone by the process of ………………
(a) Smelting
(b) Calcination
(c) Roasting
(d) Electromagnetic separation
Answer:
(d) Electromagnetic separation
Question 9.
Which one of the following is not feasible?
(a) Zn(s) + Cu²⁺(aq) → Cu(s) + Zn²⁺(aq)
(b) Cu(s) + Zn²⁺+(aq) → Zn(s) + Cu²⁺(aq)
(c) Cu(s) + 2Ag⁺(aq) → Ag(s) + Cu²⁺(aq)
(d) Fe(s) + Cu²⁺(aq) → Cu(s) + Fe²⁺(aq)
Answer:
(b) Cu(s) + Zn²⁺+(aq) → Zn(s) + Cu²⁺(aq)
Question 10.
Electrochemical process is used to extract ………………
(a) Iron
(b) Lead
(c) Sodium
(d) Silver
Answer:
(c) Sodium
Question 11.
Flux is a substance which is used to convert ………………
(a) Mineral into silicate
(b) Infusible impurities to soluble impurities
(c) Soluble impurities to infusible impurities
(d) All of these
Answer:
(b) Infusible impurities to soluble impurities
Question 12.
Which one of the following ores is best concentrated by froth – floatation method?
(a) Magnetite
(b) Hematite
(c) Galena
(d) Cassiterite
Answer:
(c) Galena
Question 13.
In the extraction of aluminium from alumina by electrolysis, cryolite is added to ………………
(a) Lower the melting point of alumina
(b) Remove impurities from alumina
(c) Decrease the electrical conductivity
(d) Increase the rate of reduction
Answer:
(a) Lower the melting point of alumina
Question 14.
Zinc is obtained from ZnO by ………………
(a) Carbon reduction
(b) Reduction using silver
(c) Electrochemical process
(d) Acid leaching
Answer:
(a) Carbon reduction
Question 15.
Cupellation is a process used for the refining of ………………
(a) Silver
(b) Lead
(c) Copper
(d) Iron
Answer:
(a) Silver
Question 16.
Extraction of gold and silver involves leaching with cyanide ion. Silver is later recovered by ………………
(a) Distillation
(b) Zone refining
(c) Displacement with zinc
(d) liquation
Answer:
(c) Displacement with zinc
Question 17.
Considering Ellingham diagram, which of the following metals can be used to reduce alumina?
(a) Fe
(b) Cu
(c) Mg
(d) Zn
Answer:
(c) Mg
Question 18.
The following set of reactions are used in refining Zirconium
This method is known as
(a) Liquation
(b) Van Arkel process
(c) Zone refining
(d) Monds process
Answer:
(b) Van Arkel process
Question 19.
Which of the following is used for concentrating ore in metallurgy?
(a) Leaching
(b) Roasting
(c) Froth floatation
(d) Both (a) and (c)
Answer:
(d) Both (a) and (c)
Question 20.
The incorrect statement among the following is ………………
(a) Nickel is refined by Monds process
(b) Titanium is refined by Van Arkels process
(c) ZinC blende is concentrated by froth floatation
(d) In the metallurgy of gold, the metal is leached with dilute sodium chloride solution
Answer:
(d) In the metallurgy of gold, the metal is leached with dilute sodium chloride solution
Question 21.
In the electrolytic refining of copper, which one of the following is used as anode?
(a) Pure copper
(b) Impure copper
(c) Carbon rod
(d) Platinum electrode
Answer:
(b) Impure copper
Question 22.
Which of the following plot gives Ellingham diagram?
(a) ∆S Vs T
(b) ∆Gº Vs T
(c) ∆Gº Vs
(d) ∆Gº Vs T
Answer:
(b) ∆Gº Vs T
Question 23.
In the Ellingham diagram, for the formation of carbon monoxide
a) `\left( \frac { \triangle { S }^{ 0 } }{ \triangle T } \right)` is negative
(b) `\left( \frac { \triangle G ^{ 0 } }{ \triangle T } \right)` is positive
(c) `\left( \frac { \triangle G ^{ 0 } }{ \triangle T } \right)` is negative
(d) initially `\left( \frac { \triangle T }{ \triangle G ^{ 0 } } \right)` is positive, after 700C, `\left( \frac { \triangle G ^{ 0 } }{ \triangle T } \right)` is negative
Answer:
(c) `\left( \frac { \triangle G ^{ 0 } }{ \triangle T } \right)` is negative
Quesion 24.
Which of the following reduction is not thermodynamically feasible?
(a) Cr₂O₃ → Al₂O₃ + 2Cr
(b) Al₂O₃ → Cr₂O₃ + 2Al
(c) 3TiO₂ + 4Al → 2Al₂O₃ + 2Al
(d) none of these
Answer:
(b) Al₂O₃ → Cr₂O₃ + 2Al
Question 25.
Which of the following is not truc with respect to Ellingham diagram?
(a) Free energy changes follow a straight line. Deviation occurs when there is a phase change.
(b) The graph for the formation of CO₂ is a straight line almost parallel to free energy axis.
(c) Negative slope of CO shows that it becomes more stable with increase in temperature.
(d) Positive slope of metal oxides shows that their stabilities decrease with increase in temperature.
Answer:
(b) The graph for the fonnation of CO₂ is a straight line almost parallel to free energy axis.
II. Answer the following questions:
Question 1.
What is the difference between minerals and ores?
Answer:
Minerals:
- Minerals contain a low percentage of metal.
- Metal cannot be extracted easily from minerals.
- Clay Al₂O₃. SiO₂. 2H₂O is the mineral of aluminium.
Ores:
- Ores contain a large percentage of metal.
- Ores can be used for the extraction of metals on a large scale readily and economically.
- Bauxite Al₂O₂ 2H₂O is the ore of aluminium.
Question 2.
What are the various steps involved in extraction of pure metals from their ores?
Answer:
- The extraction of pure metals from the concentrated ores is carried out in two
steps:
- Conversion of the ore into oxides of the metal of interest.
- Reduction of the metal oxides to elemental metals.
Question 3.
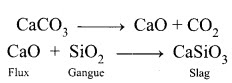
What is the role of Limestone in the extraction of Iron from its oxide Fe₂O₃?
Answer:
- In the extraction of iron, a basic flux limestone is used. Limestone decomposes to form CaO which reacts with silica gangue present in the iron ore is acidic in nature to form calcium silicate (slag).
Question 4.
Which type of ores can be concentrated by froth floatation method? Give two examples for such ores.
Answer:
- Sulphide ores can be concentrated by froth floatation method,
- Copper pyrites (CuFeS₂H₂)
- Zinc blende (ZnS)
- Galena (PbS)
Question 5.
Out of coke and CO, which is better reducing agent for the reduction of ZnO? Why?
Answer:
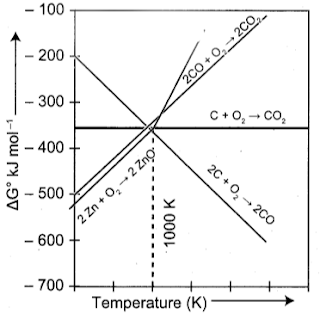
- Coke (C) is a better reducing agent for the reduction of ZnO. Because, when we use coke, the reduction can be easily carried out at 673 K. Thus Carbon (Coke) reduces zinc oxide more easily than carbon monoxide (CO). From the Ellingham diagrams, it is quite clear that the reduction of zinc oxide is more favourable using coke ∆G for the formation of carbon monoxide from carbon is more negative).

Question 6.
Describe a method for refining nickel.
Answer:
- The impure nickel is heated in a stream of carbon monoxide at around 350K. The nickel reacts with the CO to form a highly volatile nickel tetracarbonyl. The solid impurities are left behind.
- Ni (s) + 4 CO (g) → Ni(CO)₄(g)
- On heating the nickel tetracarbonyl around 460 K, the complex decomposes to give pure metal.
- Ni(CO)₄ (g) → Ni (s) + 4 CO (g)
Question 7.
Explain zone refining process with an example using the Ellingham diagram given below.
Answer:
- Zone Refining method is based on the principles of fractional crystallisation. When an impure metal is melted and allowed to solidify, the impurities will prefer to be in the molten region, i.e. impurities are more soluble in the melt than in the solid state metal.
- In this process, the impure metal is taken in the form of a rod. One end of the rod is heated using a mobile induction heater which results in melting of the metal on that portion of the rod.
- When the heater is slowly moved to the other end the pure metal crystallises while the impurities will move on to the adjacent molten zone formed due to the movement of the heater. As the heater moves further away, the molten zone containing impurities also moves along with it.
- The process is repeated several times by moving the heater in the same direction again and again to achieve the desired purity level.
- This process is carried out in an inert gas atmosphere to prevent the oxidation of metals.E
- lements such as germanium (Ge), silicon (Si) and galium (Ga) that are used as semiconductor are refined using this process.
Question 8.
1. Predict the conditions under which
(a) Aluminium might be expected to reduce magnesia.
(b) Magnesium could reduce alumina.
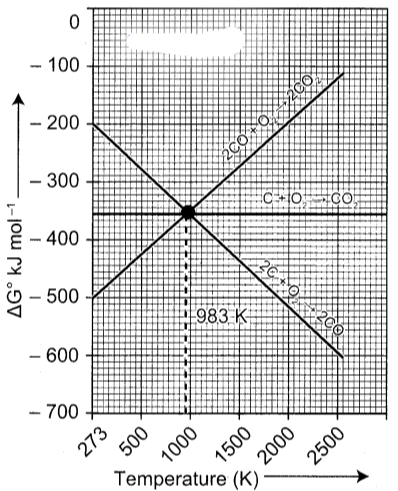
2. Carbon monoxide is more effective reducing agent than carbon below 983 K but, above this temperature, the reverse is true -Explain.
3. it is possible to reduce Fe₂O₃ by coke at a temperature around 1200 K.
Answer:
The conditions under which:
- (a) Ellingham diagram is used to predict thermodynamic feasibility of reduction of oxides of one metal by another metal. Any metal can reduce the oxides of other metals that are located above it in the Ellingham diagram. In the Ellingham diagram, for the formation of magnesia (magnesium oxide) occupy lower position than aluminium oxide. Therefore aluminium cannot be used to reduce the oxides of magnesium (magnesia). Above 1623K, A1 can reduce MgO to Mg, so that ArG⁰ becomes negative and the process becomes thermodynamically feasible.
(b)
`left( \frac { 4 }{ 3 } \right)`Al + O₂ → `left( \frac { 2 }{ 3 } \right)`Al₂O₃
2Mg + O₂ → 2MgO
- At the point of intersection of the Al₂O₃ and MgO curves in Ellingham diagram. ∆G⁰
becomes zero for the reaction:
`left( \frac { 2 }{ 3 } \right)`Al₂O₃ → 2MgO + `left( \frac { 4 }{ 3 } \right)`Al
- Below that point magnesium can reduce alumina.
2. From the Ellingham diagram, we find that at 983 K, the curves intersect.
- The value of ∆G⁰ for change of C to CO₂ is less than the value of ∆G° for change of CO to CO₂. Therefore, coke (C) is a better reducing agent than CO at 983K or above temperature. However below this temperature (e.g. at 673K), CO is more effective reducing agent than C.
3. Yes, it is possible to reduce Fe₂O₃ by coke at a temperature around 1200 K. In the Ellingham diagram, carbon line cuts across the lines of many metal oxides and hence it can reduce all those metal oxides at sufficiently high temperature. Ellingham diagram for the formation of Fe₂O₃ and CO intersects around 1000 K.
- Below this temperature, the carbon line lies above the iron line which indicates that Fe₂O₃ is more stable than CO and hence at this temperature range the reduction is not thermodynamically feasible. However above 1000 K carbon line lies below the iron line and hence we can use coke as a reducing agent around 1200 K. Around 1200 K, coke is better reducing agent because above 1000 K, Gibb’s free energy for the formation of Fe₂O₃ is more than the formation of CO₂ from C.
Question 9.
Give the uses of zinc.
Answer:
Applications of Zinc (Zn):
- Metallic zinc is used in galvanising metals such as iron and steel structures to protect them from rusting and corrosion.
- Zinc is also used to produce die-castings in the automobile, electrical and hardware industries.
- Zinc oxide is used in the manufacture of many products such as paints, rubber, cosmetics, pharmaceuticals, plastics, inks, batteries, textiles arid electrical equipment. Zinc sulphide is used in making luminous paints, fluorescent lights and x-ray screens.
- Brass an alloy of zinc is used in water valves and communication equipment as it is highly resistant to corrosion.
Question 10.
Explain the electrometallurgy of aluminium.
Answer:
Electrochemical extraction of aluminium Hall-Herold process:
- In this method, electrolysis is carried out in an iron tank lined with carbon, which acts as a cathode. The carbon blocks immersed in the electrolyte acts as a anode. A 20% solution of alumina, obtained from the . bauxite ore is mixed with molten cyrolite and is taken in the electrolysis chamber. About 10%, calcium chloride is also added to the solution. Here calcium chloride helps to lower the melting point of the mixture. The fused mixture is maintained at a temperature of above 1270 K. The chemical reactions involved in this process are as follows:
Ionisation of alumina:
- Al₂O₃ → 2Al₃ + 3OO²⁻
Reaction at cathode:
- 2Al³+ (melt) + 3e– → Al(ₗ)
Reaction at anode:
- 2O²⁻ (melt) → O₂ + 3e⁻
Since carbon acts as anode the following reaction also takes place on it.
- C (s) + O²⁻(melt) → CO + 2e⁻
- C (s) + 2O²⁻ (melt) → CO₂ + 4e⁻
Due to the above two reactions, anodes are slowly consumed during the electrolysis. The pure aluminium is formed at the cathode and settles at the bottom. The net electrolysis reaction can be written as follows:
- 4Al³⁺ (melt) + 6O² (melt) + 3C(ₛ) → 4A(ₗ ⁺ 3CO₂(g)
Question 11.
Explain the following terms with suitable examples.
Gangue
Slag
Answer:
1. Gangue:
- The impurities associated with the minerals are known as Gangue or Matrix.
2. Slag:
- A compound formed when gangue is combined with flux is called slag.
Flux + Gangue → Slag
- For example, the oxide of iron can be reduced by carbon monoxide as follows:
Fe₂O₃ + 3CO → 2Fe + 3CO₂
- In this extraction a basic flux, limestone is used.
- Since the silica gangue present in the ore is acidic in nature, the limestone combines with it to form Calcium silicate (Slag).
Question 12.
Give the basic requirement for vapour phase refining.
Answer:
The two requirements for vapour phase refining are:
- The metal should form a volatile compound with a suitable reagent.
- The volatile compound is decomposed to give the pure metal.
Question 13.
Describe the role of the following in the process mentioned.
Silica in the extraction of copper.
Cryolite in the extraction of aluminium.
Iodine in the refining of Zirconium.
Sodium cyanide in froth floatation.
Answer:
- The role of silica in the extraction of copper is to remove the iron oxide obtained during the process of roasting as slag. If the sulphide ore of copper contains iron, the silica (SiO₂) is added as flux before roasting. Then, FeO combines with silica to form iron silicate, FeSiO₃ (Slag).
- Cryolite reduces the melting point of Al₂O₃ and increases its electrical conductivity. Aluminium is produced by the electrolytic reduction of fused alumina in the electrolytic cell. Alumina is not an electrolyte. So it is made as an electrolyte by dissolving it in the fused cryolite. The function of cryolite is to lower the fusion temperature.
- Zirconium crude metal is heated with iodine in an evacuated vapour to separate from impurities and this decomposes at 1800 K to give a pure zirconium metal and iodine. Initially iodine is heated with zirconium to form a volatile compound.
- Sulphide ores which are concentrated by the froth floatation process. Depressants are used to prevent certain type of particles from forming the froth. NaCN act as a depressant to separate ZnS from PbS.
Question 14.
Explain the principle of electrolytic refining with an example.
Answer:
- The crude metal is refined by electrolysis. It is carried out in an electrolytic cell containing aqueous solution of the salts of the metal of interest. The rods of impure metal are used as anode and thin strips of pure metal are used as cathode.
- The metal of interest dissolves from the anode, pass into the solution while the same amount of metal ions from the solution will be deposited at the cathode. During electrolysis, the less electropositive impurities in the anode, settle down at the bottom and are removed as anode mud. Let us understand this process by considering electrolytic refining of silver as an example.
Cathode:
- Pure silver
Anode:
- Impure silver rods
Electrolyte:
- Acidified aqueous solution of silver nitrate. When a current is passed through the electrodes the following reactions will take place Reaction at anode.
Reaction at cathode:
Ag⁺ (aq) + 1 e⁻ → Ag (s)
- During electrolysis, at the anode the silver atoms lose electrons and enter the solution. The positively charged silver cations migrate towards the cathode and get discharged by gaining electrons and deposited on the cathode. Other metals such as copper, zinc etc.,can also be refined by this process in a similar manner.
Question 15.
The selection of reducing agent depends on the thermodynamic factor. Explain with an example.
Answer:
- From the Ellingham diagram, it is clear that metals for which the standard free energy of formation (∆⃗fG⁰) of their oxides is more negative can reduce the metal oxides for which the standard free energy of formation (∆fG⁰) of oxides is less negative.
- Thermodynamic factor has a major role in selecting the reducing agent for a particular reaction. Only that reagent will be preferred which will lead to decrease in the free energy (AG°) at a certain specific temperature.
E.g – Carbon reduce ZnO to Zn but not CO.
ZnO + C → Zn + CO …………..(1)
ZnO + CO → Zn + CO₂ ………………(2)
- In the first case, there is increase in the magnitude of ∆S° while in the second case, it almost remains the same. In other words, ∆G° will have more negative value in the first case, when C is the reducing agent then in the second case when CO acts as the reducing agent. Therefore, C is a better reducing agent.
Question 16.
Give the limitations of Ellingham diagram.
Answer:
Limitations of Ellingham diagram:
- Ellingham diagram is constructed based only on thermodynamic considerations. It gives information about the thermodynamic feasibility of a reaction. It does not tell anything about the rate of the reaction. Moreover, it does not give any idea about the possibility of other reactions that might be taking place.
- The interpretation of ∆G is based on the assumption that the reactants are in equilibrium with the product which is not always true.
Question 17.
Write a short note on electrochemical principles of metallurgy.
Answer:
- Electrochemical principles also find applications in metallurgical process. The reduction of oxides of active metals such as sodium, potassium etc., by carbon is thermodynamically not feasible. Such metals are extracted from their ores by using electrochemical methods. In this technique, the metal salts are taken in a fused form or in solution form. The metal ion present can be reduced by treating it with some suitable reducing agent or by electrolysis. Gibbs free energy change for the electrolysis process is given by the following expression
∆G⁰ = -nFE⁰
- Where n is number of electrons involved in the reduction process, F is the Faraday and E⁰ is the electrode potential of the redox couple. If E⁰ is positive then the ∆G is negative and the reduction is spontaneous and hence a redox reaction is planned in such a way that the e.m.f of the net redox reaction is positive. When a more reactive metal is added to the solution containing the relatively less reactive metal ions, the more reactive metal will go into the solution. For example,
- Cu (s) + 2Ag⁺(s) → Cu²⁺(aq) + 2Ag (s)
- Cu²⁺ (aq) + Zn (s) → Cu (s) + Zn⁺(aq)
Evaluate Yourself
Question 1.
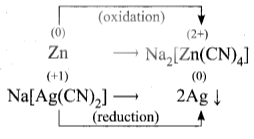
Write the equation for the extraction of silver by leaching with sodium cyanide and show that the leaching process is a redox reaction.
Answer:
- The crushed ore of argentite (Ag₂S) is leached with sodium cyanide solution. This reaction forms sodium argento cyanide
Na[Ag(CN)₂]
Step 1:
Ag₂S + 4NaCN ⇌ 2Na[Ag(CN)₂] + Na₂S
- The solution of sodium argento cyanide combines with zinc dust and forms sodium tetra cyano zincate and precipitated silver.
Step 2:
Zn + 2Na[Ag(CN)₂] → Na₂[Ag(CN)₄] + 2 Ag↓
In the step 2, redox reaction take place.
Question 2.
Magnesite (Magnesium carbonate) is calcined to obtain magnesia, which is used to make refractory bricks. Write the decomposition reaction.
Answer:
- Magnesite is a carbonate of magnesium. Magnesite when heated at 800ºC to 1000ºC at the CO₂ content in it is driven off. The residue so obtained is known as calcined magnesite.
Question 3.
Using Ellingham diagram indicate the lowest temperature at which ZnO can be reduced to zinc metal by carbon. Write the overall reduction reaction at this temperature.
Answer:
- Ellingham diagram shows variation in standard Gibbs free energy change with temperature for the formation of oxide. The Ellingham diagram shows straight line upward slope with formation of oxide, but in case of ZnO there is sudden change. Ellingham diagram helps in the selecting suitable reducing agent.
- By seeing the Ellingham diagram, the free energy formation (∆fGº) of CO from C becomes lower temperatures above 1120 K while that of CO₂ from C becomes lower above 1323 K than ∆fGº of ZnO. As ∆fGº of C0₂ from CO is always higher than that of ZnO. So C can reduce ZnO to Zn but not CO. Thus carbon is better reducing agent than CO for ZnO.
Question 4.
Metallic sodium is extracted by the electrolysis of brine (aq. NaCl). After electrolysis the electrolytic solution becomes basic in nature. Write the possible electrode reactions.
Answer:
Brine is a solution of sodium chloride (molten state):
- The process of electrolysis involves using an electric current to bring about a chemical change and make new chemicals. In the electrolysis of brine, sodium ions migrate to the cathode, where electrons enter the melt and are reduced to sodium metal.
Na⁺ + e⁻ → Na (at cathode)
- Chloride ions migrate the other way toward the anode. They give up their electrons to the anode and are oxidised to chlorine gas.
Cl ⁻ → ` \frac { 1 }{ 2 }`Cl₂ + e⁻ (at anode)
Overall reaction:
- 2NaCl → 2Na(s) + Cl₂ (g)
For aqueous solution of NaCl:
- H₂O + 2e⁻ → H₂↑+ 20H⁻ (at cathode)
Cl⁻ →` \frac { 1 }{ 2 } `Cl₂ + e⁻ (at anode)
Overall reaction:
- NaCl (aq) + H₂O(1) → Na⁺(aq) + OH⁻(aq) + H₂(g) + 12Cl₂(g)
- After electrolysis the electrolytic solution becomes basic in nature. [Due to formation of hydroxide (OH⁻) ion].


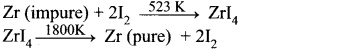




0 Comments:
Post a Comment